

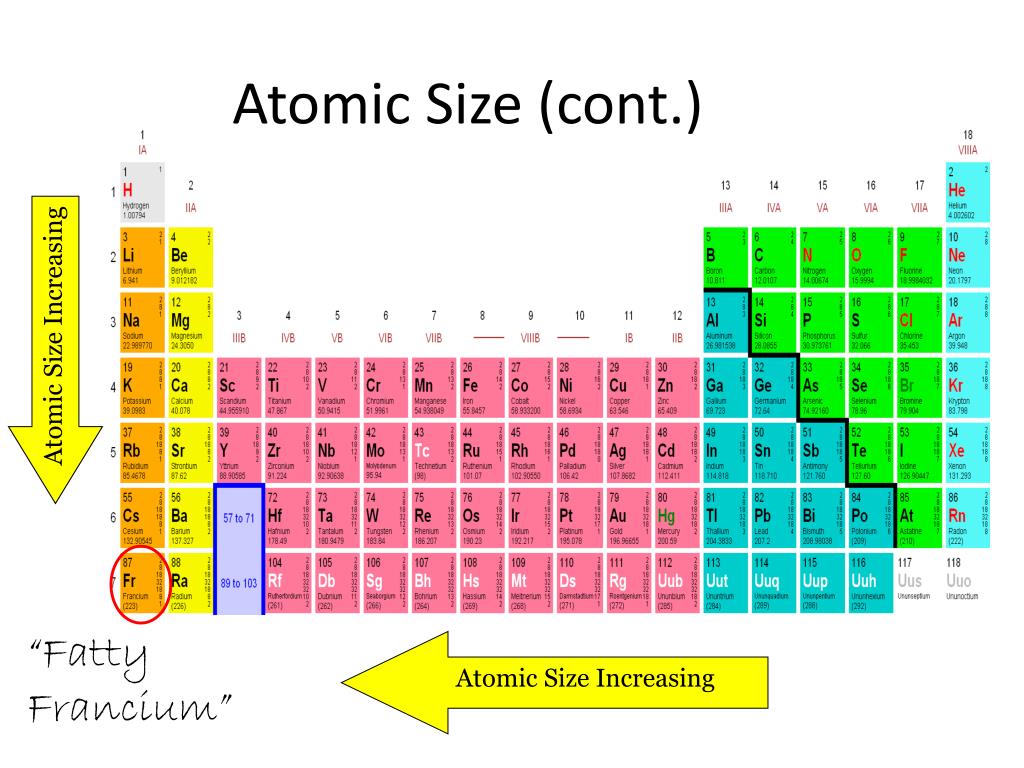
🤨 Properties that differ: One group on the periodic table is organized so that as you move down a group, the number of occupied electron shells increase. However, sodium has 11 protons (represented by its atomic number of 11), and argon has 18 protons (represented by its atomic number of 18). You could see here that both sodium and argon have a total of three occupied electron shells, following the pattern of elements in the same period. Let's compare sodium, which is the first element in period 3, with argon, the last element in period 3. 🤝 Shared Properties: In each row, the elements have the same number of occupied electron shells. This trend contributes to the differing effective nuclear charge of elements in the same group, which we'll discuss below. The atomic number, or the number of protons in an atom's nucleus, determines the basic chemical properties of said element. 🤨 Properties that differ: Going horizontally, each period is organized in order of increasing atomic number. It is also important to note that the periodic table is divided into 18 columns (called groups) and 7 rows (called periods). It was purposely made to group chemicals of similar properties together. In order to fully understand why the trends occur the way they do, it's important to cover the following topics: Organization of the Periodic TableĪs mentioned before, the periodic trends aren't too difficult to grasp since they follow the chronology of the periodic table. Foundational Concepts for Periodic Trends A cool thing about the periodic table is that it is organized to demonstrate different trends and properties of elements that can be explained by the pattern of electron configurations and the presence of electron-filled orbitals. The periodicity of the periodic table, or its tendency to recur at intervals, can help you estimate the properties of atoms that haven't even been discovered yet.įor the sake of the AP Chemistry exam, rather than only understanding the trends, you should be able to explain why they happen.


 0 kommentar(er)
0 kommentar(er)
